
A normal bacterial cell is known as a vegetative form of a bacterial cell. It can multiply freely when provided with conducive environmental conditions like the provision of nutrients, temperature, water, and oxygen. But some groups of bacteria, when exposed to unfavorable conditions such us, lack of nutrients, an insufficient supply of oxygen or CO2, lack of water and moisture, they have the ability to form a protective covering to protect themselves when they are exposed to harsh environmental conditions adapting comfortably to these conditions. This special structure is known as an endospore.
An endospore is a non-vegetative structure produced by a group of bacteria belonging to the Firmicute family. They have special characteristics that stabilize them to survive in adverse conditions for long periods of time.
These include; a phase of dormancy, a tough outer covering and they are non-reproductive forms called spores, which offer great resistance to high temperature, radiations, and chemicals like disinfectants and acids. This enables them to survive thus they are difficult to stain with basic dyes and hence a special stain is applied that uses a special dye, along with heat-steam. This staining technique is known as the Endospore stain, also known as the spore stain. It is used majorly to detect and identify the presence of a bacterial endospore and bacterial vegetative forms in a cell.
Examples of these endospore-forming bacteria include Clostridium spp and Bacillus spp. These bacteria naturally grow in soil, but they have great clinical implications by causing human bacterial infections eg Clostridium tetani causes tetanus, Clostridium botulinum produces botulin toxin which causes paralysis, Bacillus cereus causing food poisoning and Bacillus anthracis causes anthrax in cattle and humans.
Endospore staining techniques are classified based on the types of reagents used;

Endospore staining is a differential stain that aims at detecting, identifying and differentiating an endospore from the vegetative cell (an underdeveloped endospore). The principle of the role is to detect the presence or absence of the endospore, but some procedures have modified the technique by increasing the concentrations of the dyes, increasing the duration of heat fixing, application of ultraviolet radiation.
With the improved technology in microscopy, some use phase-contrast microscopy which is fast and it produces more detailed morphologies of the bacterial endospore.
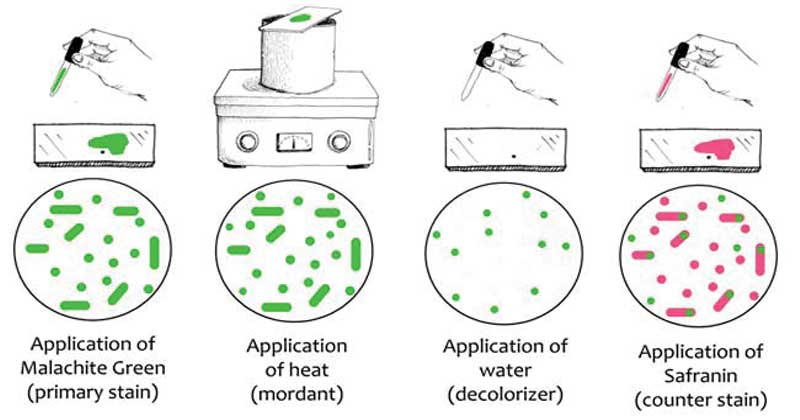
In basic Laboratories, the simplest endospore staining technique is the Schaeffer-Fulton technique because of its easy and it is rapid to identify the bacteria. It applies the use of Malachite green dye (alkaline solution with a pH of 11-11.2 and its a water-soluble dye) along with the use of steamed-heat which softens the endospore covering allowing penetration of the dye into the spore. The malachite green dye binds to the spore mildly and if washed with water, without fixing, it easily washes away, and that’s why the application of steamed heat is important to allow the dye to penetrate the endospore. Water is used as a decolorizing agent, to was away from the malachite dye from vegetative forms. Lastly, the use of a counterstain, Safranin reagent, also knows as the secondary stain, is to stain the vegetative forms of the underdeveloped Furmigates vegetative forms after the malachite dye has been washed away by the decolorizing agent (Water).
Malachite Green dye and Safranin work well in bacteria because of the alkaline nature of the Malachite Green reagents which are charged positively while the cytoplasm of the bacterial cell is basophilic hence there is an attraction between the malachite green dye with the bacterial cell, making it easier to absorb the dye. Visualization of the cells under the microscope will show the appearance of pink-red stain for the vegetative cell forms, which take up the counterstain while the endospores will appear as green dotted particles (ellipses), having taken up the Malachite green dye.
Equipment: Glass slide, Inoculation loop, Bunsen burner
The vegetative forms will take up the pink/red stain from safranin while the endospores will stain green, from the malachite green dye.
The vegetative forms stain pink/red because they take up the counterstain (Safranin) while the endospores take up the green from the Malachite green.
This is because, during smearing and heat fixing, the malachite green penetrates into the endospore with the help of the heat from the steam, and during the water-rinse, the dye is not easily washed away.
And for the vegetative forms, the dye is easily washed away because of their fragile outer covering, hence they take up the last stain which is the counterstain, hence they appear pink-red.
Decolorizing solvent (acid-alcohol)
Counterstain (Nigrosin solution)
(Preparation of microscopic slide is adapted from the above procedure)
Vegetative cells appear colorless, while endospores are red.
NOTE
Internet Sources
About Author